Measuring the impact of VZV vaccination
Varicella zoster virus (VZV) is one of the eight known human herpesviruses. Initial VZV infection results in chickenpox, while viral reactivation, following a period of latency, manifests as shingles. Separate vaccines exist to protect against both initial infection and subsequent reactivation. Controversy regarding chickenpox vaccination is contentious with most countries not including the vaccine in their childhood immunization schedule due to the hypothesized negative impact on immune-boosting; where VZV reactivation is suppressed through exogenous boosting of VZV antibodies from exposure to natural chickenpox infections. Population-level chickenpox and shingles notifications from Thailand, a country that does not vaccinate against either disease, were previously fitted with mathematical models to estimate rates of VZV transmission and reactivation. Here, multiple chickenpox and shingles vaccination scenarios were simulated and compared to a model lacking any vaccination to analyze the long-term impacts of VZV vaccination. As expected, simulations suggested that an introduction of the chickenpox vaccine, at any coverage level, would reduce chickenpox incidence. However chickenpox vaccine coverage levels above 35% would increase shingles incidence under realistic estimates of shingles coverage with the current length of protective immunity from the vaccine. A trade-off between chickenpox and shingles vaccination coverage was discovered, where mid-level chickenpox coverage levels were identified as the optimal target to maximally reduce both chickenpox transmission and shingles reactivation. Only in scenarios where shingles coverage eclipsed 90% or the shingles vaccine provided lifelong immunity, could large reductions in both chickenpox and shingles be achieved.
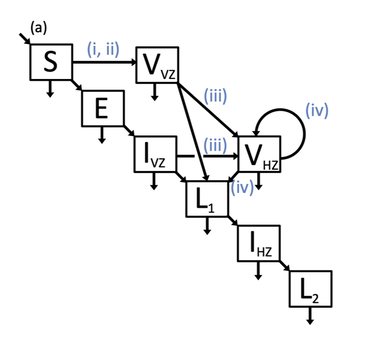
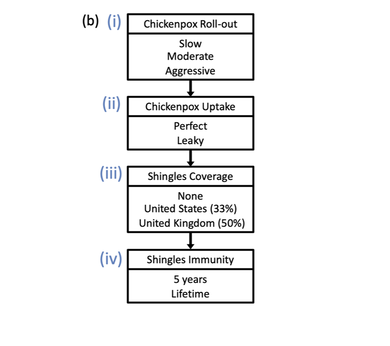
(A) Model schematic, which includes susceptible (S), exposed (E), vaccinated against chickenpox V_{VZ}, infected with chickenpox I_{VZ}, vaccinated against shingles V_{HZ}, recovered from chickenpox L_1, infected with shingles I_{HZ}, and recovered from shingles L_2 classes. Births entered the model through the S class and either remained or were moved to the vaccinated against chickenpox V_{VZ} class depending on chickenpox vaccination (i, ii). Natural death occurred in all classes and is indicated by an arrow leaving each class at the bottom. (B) Vaccination simulations varied by chickenpox vaccination roll-out (i), chickenpox vaccination uptake (ii), shingles coverage (iii), and length of shingles immunity (iv). A model without any vaccination was also simulated where all values (i)-(iv) were set to 0.
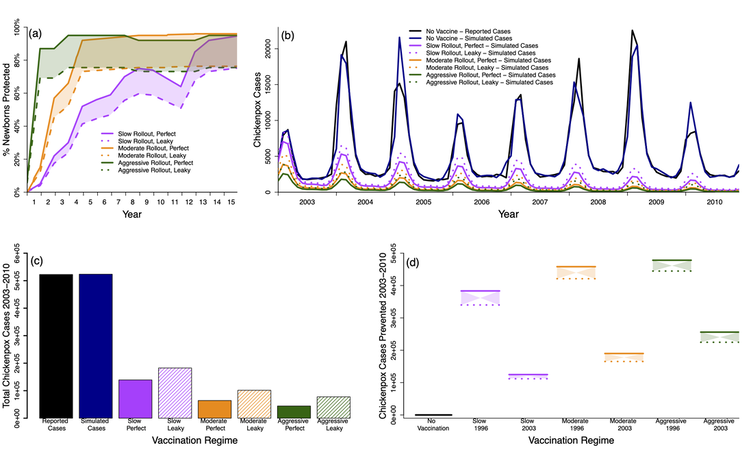
Best fit model simulations under various immunization approaches. For all panels; reported chickenpox cases (black), simulated chickenpox cases without vaccination (blue), simulated chickenpox cases utilizing the data from the (slow) 1984 measles vaccine roll-out in Thailand (magenta), simulated chickenpox cases utilizing the data from the (moderate) 1992 hepatitis B, 3rd dose vaccine roll-out in Thailand (orange), and simulated chickenpox cases utilizing the (aggressive) 2006 Japanese Encephalitis roll-out in Thailand (green). (a) Uptake levels for the 3 roll-outs with perfect (solid line) and leaky (dashed line) uptake. Colored regions between dashed and solid lines represent realistic ranges of coverage. (b) Time series of simulated cases under various immunization roll-outs, during our study period (2003-2010) if vaccination had started in 1996. (c) Reported, simulated, and immunization estimates for total chickenpox cases over our 8 year study period (2003-2010). (d) Chickenpox cases prevented under various conditions, including if vaccination had started in 1996 or 2003. X-axis labels represent vaccine coverage, vaccine start date, with dashed lines representing leaky vaccines and solid representing perfect vaccines.
|
|
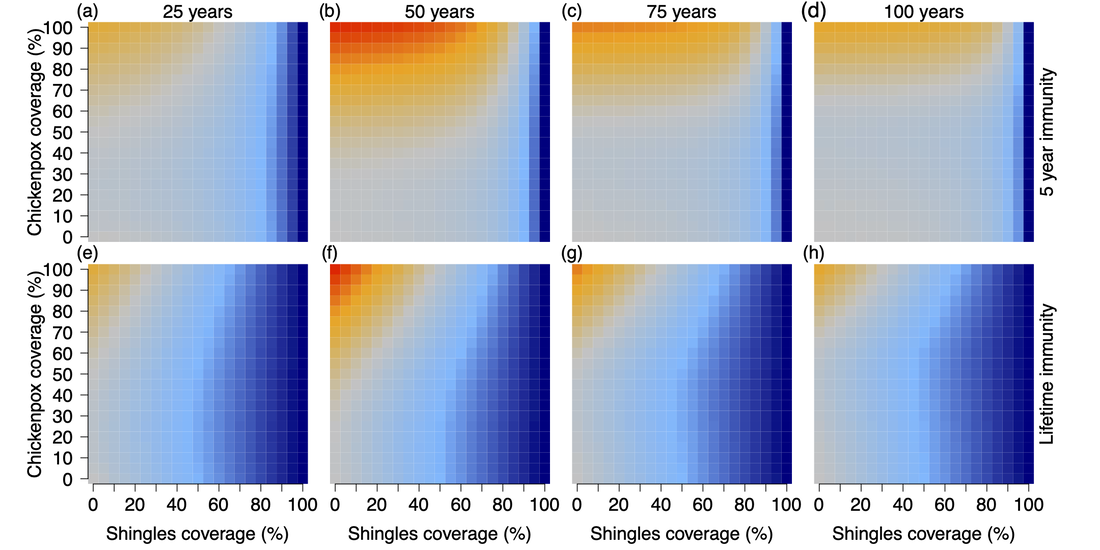
Percent change in shingles cases compared to no vaccination. Top row (a-d) simulations provided 5 years of immunity from shingles vaccination and bottom row (e-h) simulations provided lifetime immunity from shingles vaccination. Columns represent the number of years after vaccine introduction; (a & e) 25 years, (b & f) 50 years, (c & g) 75 years, and (d & h) 100 years. Shingles and chickenpox vaccine coverage (%) are shown on the x- and y-axes. Color scale on the right indicates the largest decrease in shingles cases can be seen in dark blue, while the largest increase in shingles cases can be seen in red.
|
|
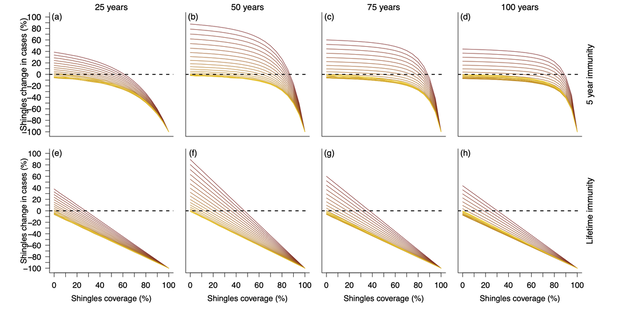
Cross section of the above figure. Top row represents simulations with 5 years of shingles vaccine immunity and the bottom row represents simulations with lifetime shingles vaccine immunity. Each column visualizes vaccination impact after 25 (left), 50 (middle left), 75 (middle right), and 100 (right) years. X-axes represent the shingles vaccination coverage, while the y-axes represents the change in shingles cases from the null vaccination simulation. Colors represent chickenpox vaccination coverage with a gradient from no vaccination (yellow) to fully vaccinated (dark red). Dotted line at 0 identifies where there would be an increase (above the line) or decrease (below the line) in shingles cases compared to the simulation lacking vaccination.
|
|
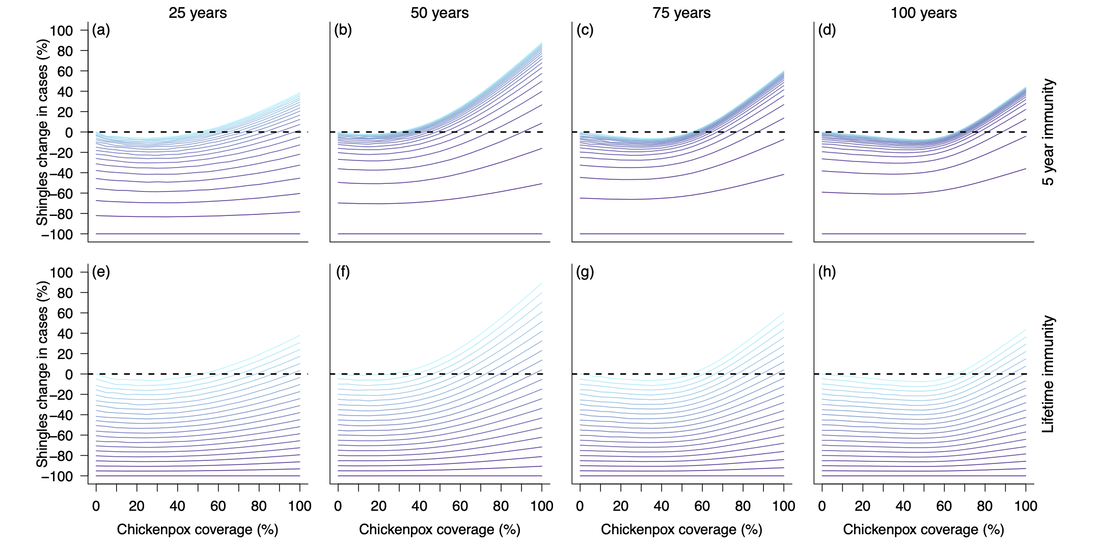
Cross section of the heatmap two figures above. Top row represents simulations with 5 years of shingles vaccine immunity and the bottom row represents simulations with lifetime shingles vaccine immunity. Each column visualizes vaccination impact after 25 (left), 50 (middle left), 75 (middle right), and 100 (right) years. X-axes represent the chickenpox vaccination coverage, while the y-axes represents the change in shingles cases from the null vaccination simulation. Colors represent shingles vaccination coverage with a gradient from no vaccination (light blue) to fully vaccinated (dark purple). Dotted line at 0 identifies where there would be an increase (above the line) or decrease (below the line) in shingles cases compared to the simulation lacking vaccination.
|