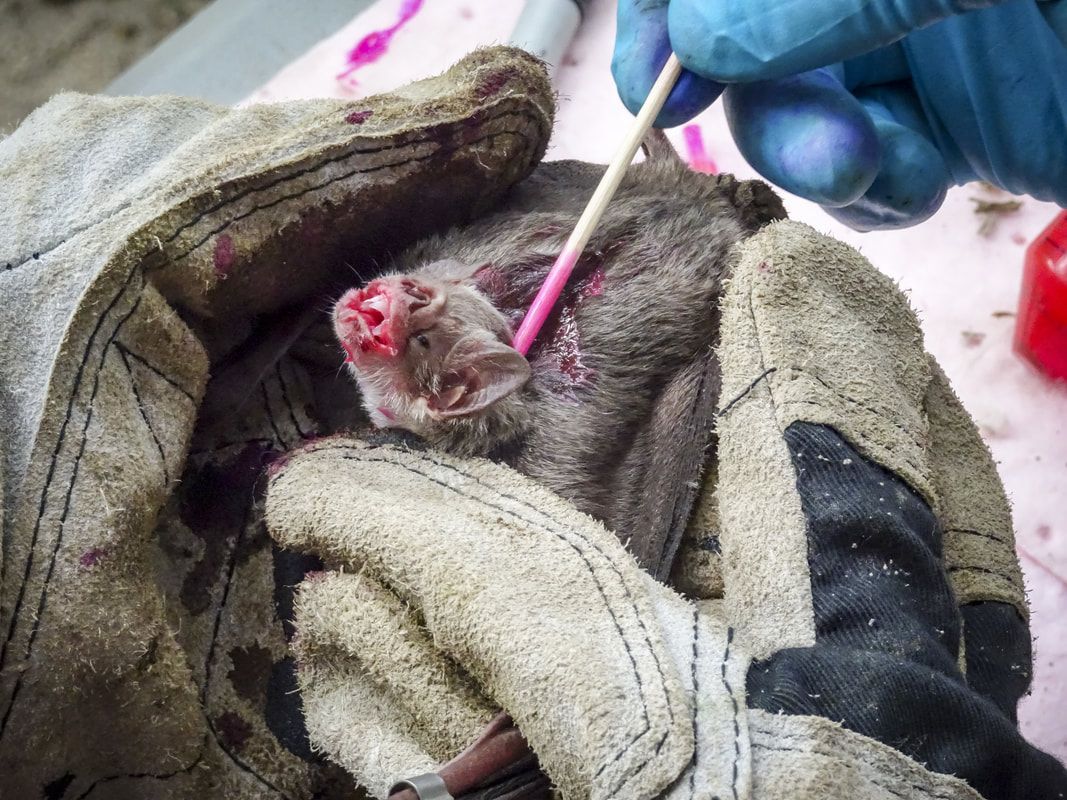
The first portion of this work was published in Dec 2019 in Nature Ecology & Evolution. A short follow up, discussing the need for increased surveillance to understand the true burden of rabies was also published in Nature Ecology & Evolution. A second manuscript examining spatiotemporal rabies dynamics with vaccines and policy is in prep (see below).
Vaccines that autonomously transfer among individuals have been proposed as a strategy to control infectious diseases within inaccessible wildlife populations. However, rates of vaccine spread and epidemiological efficacy in real-world systems remain elusive. Here, we investigate whether topical vaccines that transfer among individuals through social contacts can control vampire bat rabies—a medically and economically important zoonosis in Latin America. Field experiments in three Peruvian bat colonies, which used fluorescent biomarkers as a proxy for the bat-to-bat transfer and ingestion of an oral vaccine, revealed that vaccine transfer would increase population-level immunity up to 2.6 times beyond the same effort using conventional, non-spreadable vaccines. Mathematical models showed that observed levels of vaccine transfer would reduce the probability, size and duration of rabies outbreaks, even at low but realistically achievable levels of vaccine application. Models further predicted that existing vaccines provide substantial advantages over culling bats—the policy currently implemented in North, Central and South America. Linking field studies with biomarkers to mathematical models can inform how spreadable vaccines may combat pathogens of health and conservation concern before costly investments in vaccine design and testing.
Vaccines that autonomously transfer among individuals have been proposed as a strategy to control infectious diseases within inaccessible wildlife populations. However, rates of vaccine spread and epidemiological efficacy in real-world systems remain elusive. Here, we investigate whether topical vaccines that transfer among individuals through social contacts can control vampire bat rabies—a medically and economically important zoonosis in Latin America. Field experiments in three Peruvian bat colonies, which used fluorescent biomarkers as a proxy for the bat-to-bat transfer and ingestion of an oral vaccine, revealed that vaccine transfer would increase population-level immunity up to 2.6 times beyond the same effort using conventional, non-spreadable vaccines. Mathematical models showed that observed levels of vaccine transfer would reduce the probability, size and duration of rabies outbreaks, even at low but realistically achievable levels of vaccine application. Models further predicted that existing vaccines provide substantial advantages over culling bats—the policy currently implemented in North, Central and South America. Linking field studies with biomarkers to mathematical models can inform how spreadable vaccines may combat pathogens of health and conservation concern before costly investments in vaccine design and testing.
Ongoing work: There is a bit more complexity to vaccine/vampiricide spread and rabies transmission dynamics than a single colony. I am currently working on expanding our model to include metapopulation dynamics. This model will examine bat movement between colonies and test various intervention strategies, such as a mix of culling (vampiricide & vaccination) with immunization, which may be ring immunization, random vaccination, large colony-size focused vaccination, or creating a wall of immunized colonies.
|
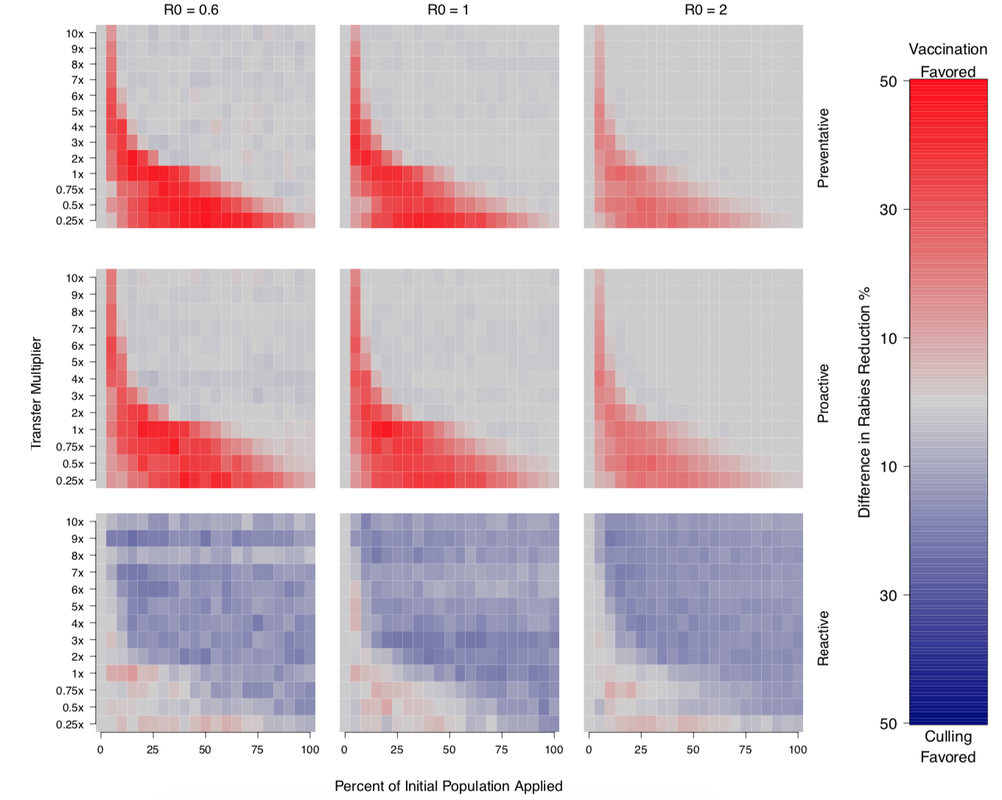
Below is the comparison between vaccination and vampiricide under various intervention strategies (preventative, proactive, reactive) and rabies R0 values. Vampiricide, which has been the favored rabies control agent for decades, is only favored in a few scenarios (blue squares), while vaccination (red squares) is typically more beneficial.
|
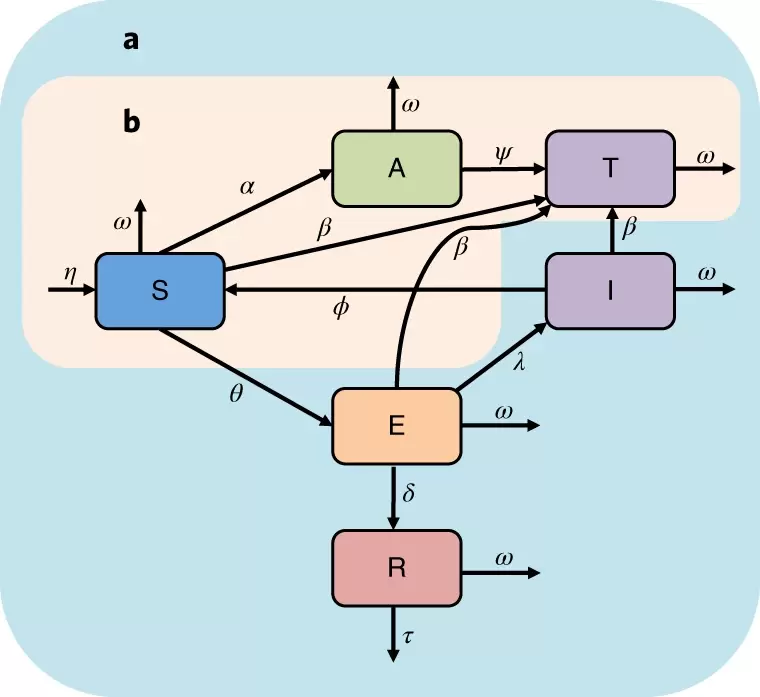
At Left: Our model, includes both vaccine/vampiricide and rabies transmission. Susceptible individuals are applied with orotopical poison (vampiricide) or vaccine and then released back into their colony where they can spread it to others. Alternatively a susceptible bat can come in contact with a rabid bat and become exposed, where they may develop rabies or natural immunity.
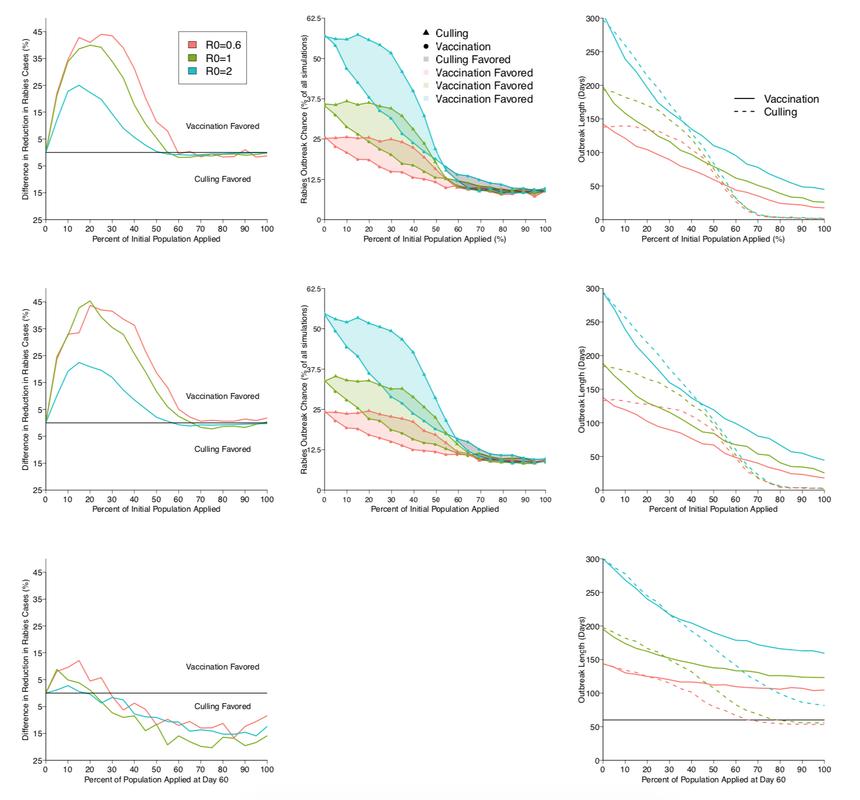
Below: Effects of preventative (top row), proactive (middle row), and reactive (bottom row) strategies of culling and vaccination on rabies outbreaks. The first column shows the difference in the reduction of rabies cases; y-values above 0 favor vaccination and below 0 favor culling. The second column is the chance of a rabies outbreak under various conditions over 1000 simulations. For each R0 value (blue, green, red) at various initial application levels (x-axis), for each culling (triangles) and vaccination (circles), the chance of a rabies outbreak is shown. Shaded regions represent the difference between vaccination and culling, with culling favored in grey regions and vaccination favored in blue, green, or red regions. The probability of outbreaks was not modeled for reactive control since, by definition, outbreaks had already occurred. The third column shows the length of a rabies outbreak under various R0 conditions for both vaccination and culling. The horizontal line in the bottom right panel is at day 30, the first day control measures were implemented.
|
Pages |
© COPYRIGHT 2023. ALL RIGHTS RESERVED.
|